Implementation of Single-Use in Drug Substance filling before transportation: Product Development case study.
The development of a new product or service is better done as a collaboration between the developer and the potential end user/s. Systems developed in isolation often end up answering questions no one has ever asked, or fails to develop a full solution. With that in mind the enclosed article discusses the product development cycle of a system for bulk filtration of Active Pharmaceutical Ingredients (API’s).

How this development progressed from Generation One to Generation Two and the lessons learned along the way.
It is interesting to think that one of the first unit to use Single-Use technology in bioprocessing is the one that has been among the last to benefit from Single-Use automation. The step in question is the bulk filtration of product prior to shipping. Twenty plus years ago this was already a Single-Use step, we just did not think of it in that way. Today in many cases we are still performing this process in much the same way we did prior to the introduction of Single-Use technology.
If we go back to the days prior to Single-Use being widely adopted; the bulk filling step involved a stainless steel tank containing the product being wheeled to a filling suite. Then a connection via a steam-able valve onto silicon tubing (which would have been autoclaved), through a pump to drive a filtration process. The critical filling step being completed in a laminar flow hood under Class 100 (ISO 5) conditions into a sterile plastic bottles, sat on a balance. The final step to fill the bottle involved an operator removing the lid and in effect performing an open filling step. With regards to automation perhaps the pump was operated by use of a foot pedal. Apart from replacing the stainless steel tank with a bag and the steam cross with aseptic connectors the process as performed today has changed very little.
A customer approached Parker and asked for our help in developing a solution that would address a number of concerns related to this unit operation.
1. Customer Concerns
- Filling High Potency Active Pharmaceutical Ingredients API’s (HPAPI) to eliminate any open processing to protect the operator and the product.
- The need to standardise the filling platform with a view to Standard Operating Procedure (SOP) writing, thereby simplifying training and eliminating variation from the process.
- Reduce the number of people involved directly in the filling step, in order to minimise the people in a clean room during a critical operation.
In collaboration with the customer, Parker developed the Generation One SciLog SciFlex Filter and Dispense (see fig.1). The SciLog SciFlex Filter and Dispense was able to address all of the above concerns.
The system allowed fully enclosed automated bulk filling of the HPAPI into bottles in preparation for transportation to a filling site. Working with the customer, we were able to identify a number of benefits of automated closed filling.
1.1 The elimination of false positives resulting in the predictable release of product.
A false positive brings with it an inevitable quarantine and investigation process, all of which takes time. It can be difficult to assign a value to this beyond the cost of completing a deviation investigation and review. In terms of administration costs alone, it has been reported at a starting figure of €2,000.00, no doubt rising to tens of thousands and beyond if the deviation results in rework being required or, in the most extreme cases, the batch being rejected.
1.2 Time saved compared to the manual process
We were also able to generate data on time savings based on two filling volumes and a set batch size. If these savings were applied to a facility producing 35 batches per year the time saving and therefore cost reductions would become significant, about €100,000’s.

1.3 Reduction in clean room personal required to complet of the process
The need for QC sampling and monitoring, along with QA oversight on documentation is reduced, as the process is fully enclosed and automated. As a result, the number of people required to be in the clean room is reduced. Resulting in a reduction in complexity.
1.4 Standardisation drives simplification

With a standard platform, training and SOP writing become simpler. As a result, operators perform the task to consistent standards reducing deviations and the risk of operator’s error. The consumables used in the process are standardised and as a result simplify supply chain and handling process; again reducing the risk of operator’s error.
At this stage in the product life cycle Parker was able to provide a solution that put liquid into bottles, on a standardised platform that use fully enclosed automated filling processes. This became the generation One SciLog SciFlex Filter and Dispense. As a producer of equipment we thought our responsibility ended at this point. What we learned during conversations with our customer was that putting liquid in bottles was not the end point. What mattered was achieving the required outcome, namely the safe arrival of the bottles at the filling site. What we had not looked at but what was needed was what happens to the bottle after it had been filled in.
Customer feedbacks drove the development of Generation two (see Figure 2), but critically this was not just focused on hardware.
2. Working with our customer, base we identified rooms for improvement
2.1 Increase the filling accuracy
The filling accuracy on the Generation one was of +/- 10%. This was initially deemed to be acceptable as the process was bulk fill. However, feedback from the Quality Assurance team at the customer site leads us to increase the filling accuracy to a much higher level of +/-10 mg in 1,000 mg. What drove this was the knowledge of how many bottles they needed to fill as well as product and consumable reconciliation. In Generation One the filling accuracy was based on a load cell reading off the skid. In the Generation Two the load cell is located under the receiving vessel. With the Generation 1 system based on a 20L and a 500ml fill there could be anywhere between 36 and 44 bottles to be filled all of which need to be prepared and accounted for either filled bottles or empty and discarded bottles. With the improvements made to the Generation Two system, the maximum number of bottles required is 40 while the minimum is 39.
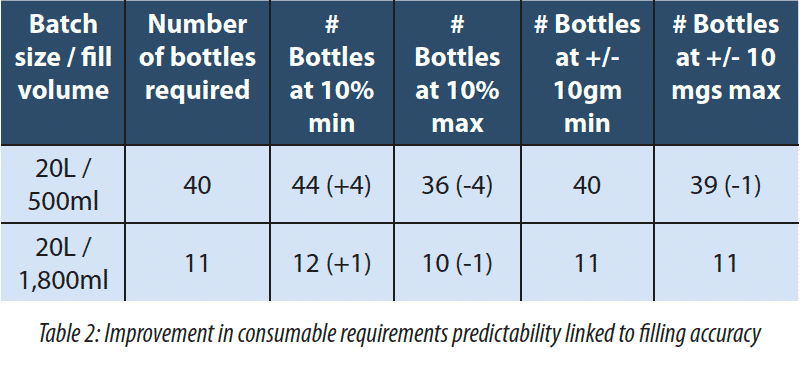
2.2 The Improvement of the filling process can be assessed in a number of ways.
- Speed of filling. This can be controlled by the end user. What determines the filling speed is the balance between minimising the production of foam (slower is better), accuracy of fill (slower is better), reducing shear exposure (slower is better), running the most efficient process possible (faster is usually better). The Generation two system allows for a rapid (based on foaming and shear information) fill to 90% of target volume, then the process can be run at a slower rate to ensure accuracy of the fill. Thereby balancing the requirement for product quality and process efficiency.
- Liquid dropped in to a bottle vertically from a cap will result in foam. So to avoid this a slow filling rate is required. This foaming can be mitigated by running the liquid down the side of the bottle thus allowing for a faster filling rate. To facilitate this, Parker developed a J- tube system which diverts the flow of liquid to the bottle side. The design and effect of the J tube configuration can be seen in Figures 3a, 3b and 3c.

2.3 Process facility knowledge should drive component selection.
A Single-Use can consist of many materials; Platinum cured silicon and or Thermoplastic elastomer (TPE) for tubing, Polycarbonate or PETG bottles, Polypropylene or PVDF fittings to name but a few. These materials need to work at a specific range of temperatures. For example, bottles may be filled under ambient conditions with the solution being filled coming out of cold storage at say 4 °C, this presents little or no challenge from a material compatibility point of view.
However, take those materials and store them in dry Ice at -78.5 °C, now the choice of materials becomes critical. PETG has a lower temperature specification of -40°C while the Polycarbonate specification is -135°C. TPE becomes brittle at around -40°C while Silicon retains its elastic properties well below the -78°C seen under dry ice storage.
Knowing the full extent of the storage conditions the product will be subjected to across the supply chain means this choosing materials that can support that process. However, not all products are compatible with all materials used to construct a Single-Use assembly. For example, a protein may bind to platinum cured silicon, meaning it cannot be used in an assembly. In that instance there may be no choice but to use a TPE tubing. So long as the end user has disclosed the low temperature shipping process the necessary safeguards around handling and manifold support can be put in place to protect the product.
2.4 Validate the system.
The SciLog SciPure FD was designed and has been validated to perform the fully automated and contained filtration and dispense of bulk API’s. As a piece of hardware you could consider this as validated. However, the function of the Parker equipment designed is to place liquid into bottles for shipping. So, there are two parts to this process: the filtration and dispensing of product followed by the shipping to the final destination. If the shipping cannot be completed successfully we don’t have a solution, merely a part of a solution. As a result of this feedback Parker, completed a shipping study to ASTM D4169 (See table 3).
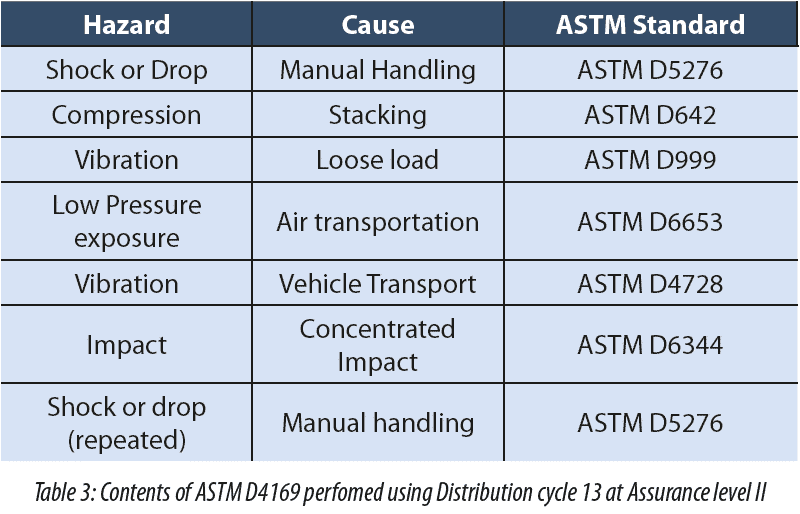
In addition to the testing the bottles were subjected to further testing to demonstrate the post shipping integrity of the bottles.
It is only after completing this study that we could claim to have a system for the filtration, dispensing and shipping of bulk API’s.
Conclusion
During product development unless you are able to collect feedback from a wide range of stakeholders, you run the risk of developing a solution that is either incomplete or worse still one that answers a question that has never been asked. It was only by working with our partner that Parker was able to identify what truly mattered in this process beyond the functionality of the system.
- Filling accuracy to support process predictability and consumable use
- Delivery of liquid to the bottle to optimise the process time
- Elimination of foaming
- The importance of understanding the full supply chain conditions and the impact that it has on material selection
- Understanding what the true outcome is. In this case not just putting liquid into bottles but the safe arrival of those bottles at a fill finish facility
Taking all these factors into account drove the development of the SciLog SciPure FD hardware and the validation package that supports the whole process. It was only by doing this that we were able to bring all the advantages of Single-Use technology and Single-Use technology automation to bear on this critical step to provide a solution that was focused on the required outcomes.
 |
Guy MATTHEWS – PARKERGuy Matthews is the Global Market Development Manager at Parker Bioscience based at the manufacturing site in Birtley County Durham UK. Guy is Microbiologist by training and has worked in Biopharma for all his career. Starting out as a Scientist at a well-known CMO in the UK before moving in to more commercial roles within the industry.
|
The original of this case study was published here: https://a3p.org/implementation-of-single-use-in-drug-substance-filling-before-transportation-product-development-case-study/
